The device is a flexible plastic ring which is placed into the vagina. The NuvaRing releases the hormones progestin and estrogen into the body, which prevents the ovaries from producing mature eggs.
A NuvaRing is typically worn for three weeks, after which it should be removed - to allow menstruation. A regular menstrual period will typically start within two or three days of removing the ring. After a one week break a new ring can be inserted.
NuvaRing is manufactured by the pharmaceutical giant Merck. It was first approved in Holland on February 14, 2001, and months later 14 other European Union countries approved.
On October 3, 2001, the U.S. Food and Drug Administration (FDA) made NuvaRing the only vaginal hormonal contraceptive available in the country, and it was first marketed in 2002.
Over 1.5 million women around the world use NuvaRing as a means of contraception.
Merck claims that the NuvaRing works just as well as the Pill - being 99 percent effective in preventing pregnancy.
A doctor's prescription is required to buy a NuvaRing.
It's particularly important to note that NuvaRing does not protect against HIV infection and other sexually transmitted diseases (STDs).
How does the NuvaRing work?
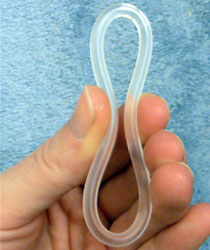
The NuvaRing is very flexible
The NuvaRing is a small ring that is easy to insert and remove.It is vital to properly position the ring in the vagina for it to be effective. The ring must not move around - it should stay in place once inserted.
How to insert a NuvaRing
1. Wash and dry your hands.
2. Hold the Nuvaring between your thumb and index finger
3. Press the sides of the ring together.
4. Either lie down, squat or stand up on one leg (whichever is most comfortable).

5. Carefully push the ring inside the vagina as far as you can - do not worry about pushing it in too deep as the ring cannot pass the cervix.

6. Keep the NuvaRing in place for three weeks.
How to remove a NuvaRing
Remove the ring after it has been in place for three weeks. To remove it, simply hook your index finger under the forward rim and pull the ring out.

Who should not use a NuvaRing?
Women are strongly advised to talk with a health care professional before using this form of contraception.Smoking severely increases the risk of serious cardiovascular side effects if you are on combination oral contraceptives, such as NuvaRing.
Women should not use the NuvaRing if they:
- Have a history of either: heart attack, stroke, blood clots in their legs (thrombophlebitis), lungs (pulmonary embolism), eyes, or DVT (deep vein thrombosis).
- Are allergic to any of the components of the NuvaRing
- Have heart valve or heart rhythm disorders
- Have a liver tumor
- Suffer from very high blood pressure
What are the risks and side effects of NuvaRing?
NuvaRing secretes hormones which can cause major changes in the blood clotting system. Using a NuvaRing can potentially allow blood to clot more easily.It is uncertain whether the risk of blood clots is greater with NuvaRing compared to birth control pills - which secrete the same hormones.
In fact, thirty-seven lawsuits were filed in Essex County, N.J., Superior Court and a federal court in Newark, N.J. against the manufacturer of NuvaRing, because the device caused blood-clot complications that resulted in amputations, pulmonary embolisms and even death.
In addition to an increased risk of blood clots, other risks associated with NuvaRing, include:
- Strokes and heart attacks - NuvaRing can increase the risk of strokes or heart attacks. Smoking while using NuvaRing further increases the risk of stroke or heart attack.
- High blood pressure - like other combination hormonal contraceptives, NuvaRing can elevate blood pressure levels.
- Cancer - There is a small small increase in the risk of cancer of the reproductive organs and breast associated with using NuvaRing.













0 comments:
Post a Comment